EX937
Our Breakthrough Solution for Refractory Chronic Cough
Refractory chronic cough is a debilitating condition that affects approximately 10% of the global population, significantly impacting quality of life. Despite its widespread prevalence, there are no FDA-approved treatments available, leaving patients with limited options and ongoing discomfort. We are pioneering an innovative solution with EX937, a proprietary small molecule developed to target the underlying mechanisms of chronic cough.
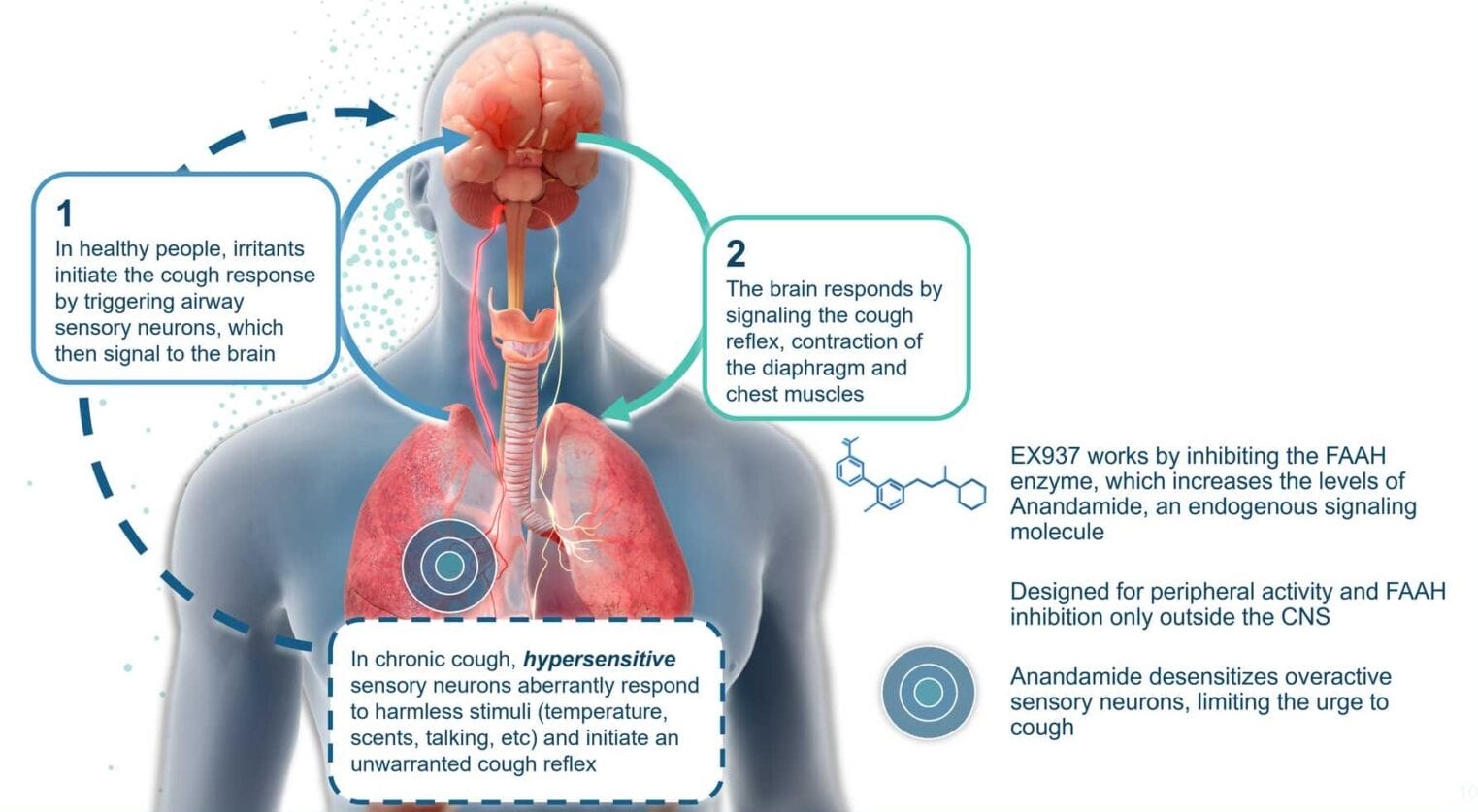
How EX937 Is Designed to Work
EX937 works by peripherally inhibiting the FAAH (fatty acid amide hydrolase) enzyme, which regulates levels of anandamide, a key molecule involved in sensory processing and pain modulation. By increasing anandamide levels, EX937 helps reduce neuronal hypersensitivity, which is believed to drive chronic cough. This novel approach offers a targeted, effective and well-tolerated solution to an urgent unmet medical need.
Key EX937 Highlights
- IND-enabling studies and pre-IND meeting completed
- Once-a-day oral dosing
- Demonstrated efficacy and favorable safety by being entirely excluded from the central nervous system and brain
- Planned Phase 1 study to be conducted in Australia providing regulatory and financial benefits
Demonstrated Efficacy and Safety
In preclinical models, EX937 has shown robust efficacy in modulating chronic cough while maintaining a favorable safety profile. Unlike therapies that impact the central nervous system, EX937 is designed to act peripherally, reducing the risk of brain-related side effects and providing a potentially safer treatment option for patients.
With EX937, we aim to redefine what is possible in chronic cough treatment, bringing innovation to an area that has been overlooked for too long.
- Worley et al. (2017). European Respiratory Journal, 50: 1700782
- Unpublished data from large pharma partner
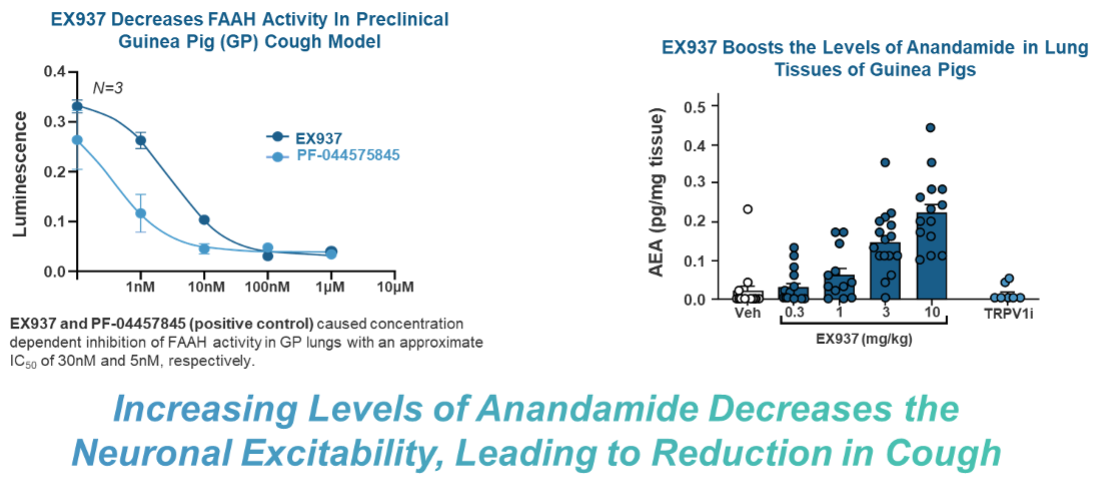
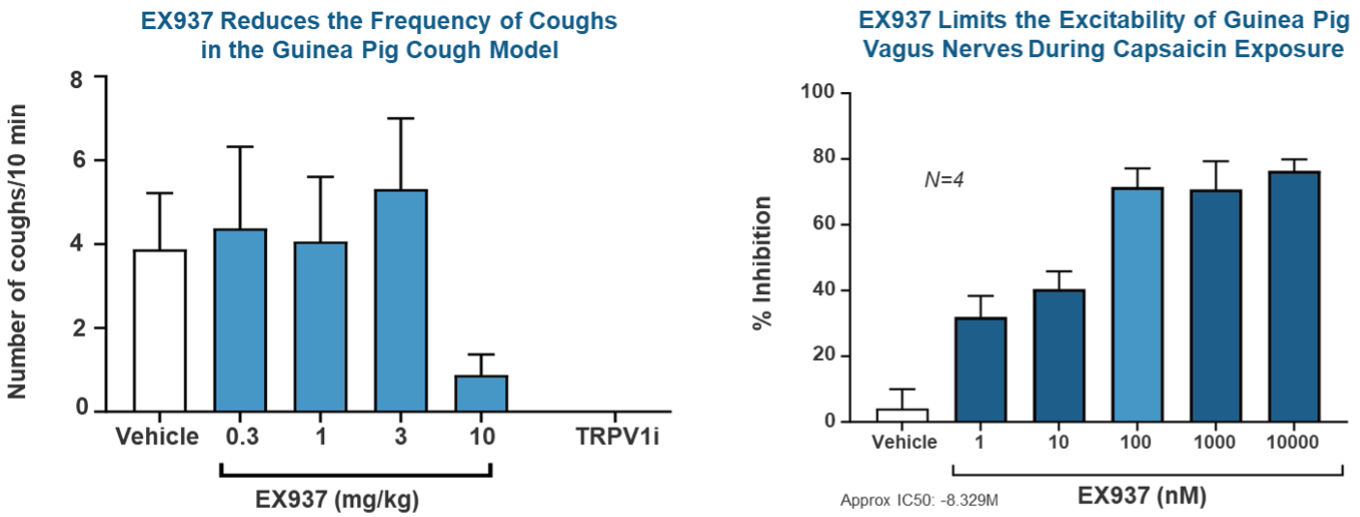
Demonstrated Efficacy
Using Capsaicin, a potent compound found in chili peppers, to induce cough, we have found that EX937 is effectively able to reduce the number of coughs in guinea pig cough models at a dose of 10 mg/kg
- Meltzer, E. O. (2021). Prevalence and Burden of Chronic Cough in the United States. The Journal of Allergy and Clinical Immunology, 9(11), 4037–4044.
- Lee JH et. al. Epidemiology of adult chronic cough: disease burden, regional issues, and recent findings. Asia Pac Allergy. 2021 Oct 18;11(4):e38. doi: 10.5415/apallergy.2021.11.e38. PMID: 34786368; PMCID: PMC8563099.
- Source: Transparency Market Research
Refractory Chronic Cough Is A Significant Unmet Need With No FDA-Approved Drug
Refractory chronic cough affects approximately:

- Meltzer, E. O. (2021). Prevalence and Burden of Chronic Cough in the United States. The Journal of Allergy and Clinical Immunology, 9(11), 4037–4044.
- Lee JH et. al. Epidemiology of adult chronic cough: disease burden, regional issues, and recent findings. Asia Pac Allergy. 2021 Oct 18;11(4):e38. doi: 10.5415/apallergy.2021.11.e38. PMID: 34786368; PMCID: PMC8563099.
- Source: Transparency Market Research
Potential Future Indications

Hyperactive Bladder
$4.2 Billion Market1

Peripheral Neuropathic Pain
$4 Billion Market1
Migraine Headache
$2.2 Billion Market1
Key Scientific EX937 Publications
Our Programs
EX937
EX937 is an innovative small molecule being developed for the treatment of refractory chronic cough, a condition that affects approximately 10% of the global population. Leveraging a novel and differentiated approach, by peripherally inhibiting the FAAH enzyme, EX937 has demonstrated marked efficacy and favorable safety in preclinical models.
EX14280 and EX14663
These globally active FAAH inhibitors leverage a chemical scaffold similar to EX937 to achieve body-wide enhancement of anandamide-mediated signaling. Substantial preclinical and clinical data with these and other FAAH inhibitors indicate potential usefulness in the treatment of Social Anxiety Disorder and Autism Spectrum Disorder.
