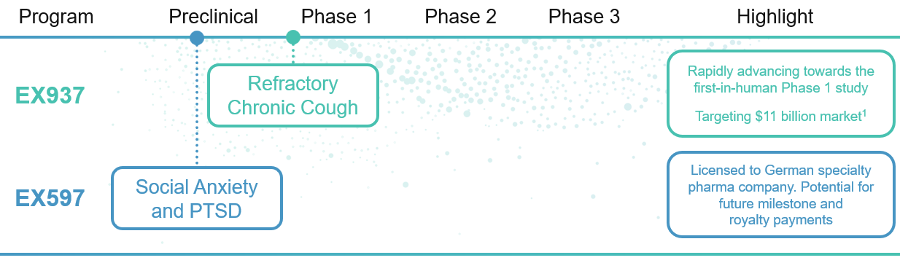
Our lead program, EX937, is a first-in-class, proprietary small molecule being developed for refractory chronic cough, a large underserved market with no current FDA-approved therapies, that affects approximately 10% of the global population. EX937 also has the potential to be utilized across a number of blockbuster indications, including hyperactive bladder, peripheral neuropathic pain and migraine headaches. Our follow-on program, EX597, is licensed to and being developed by a specialty German Pharma company for the treatment of social anxiety and PTSD.